Math please check and thank you very much
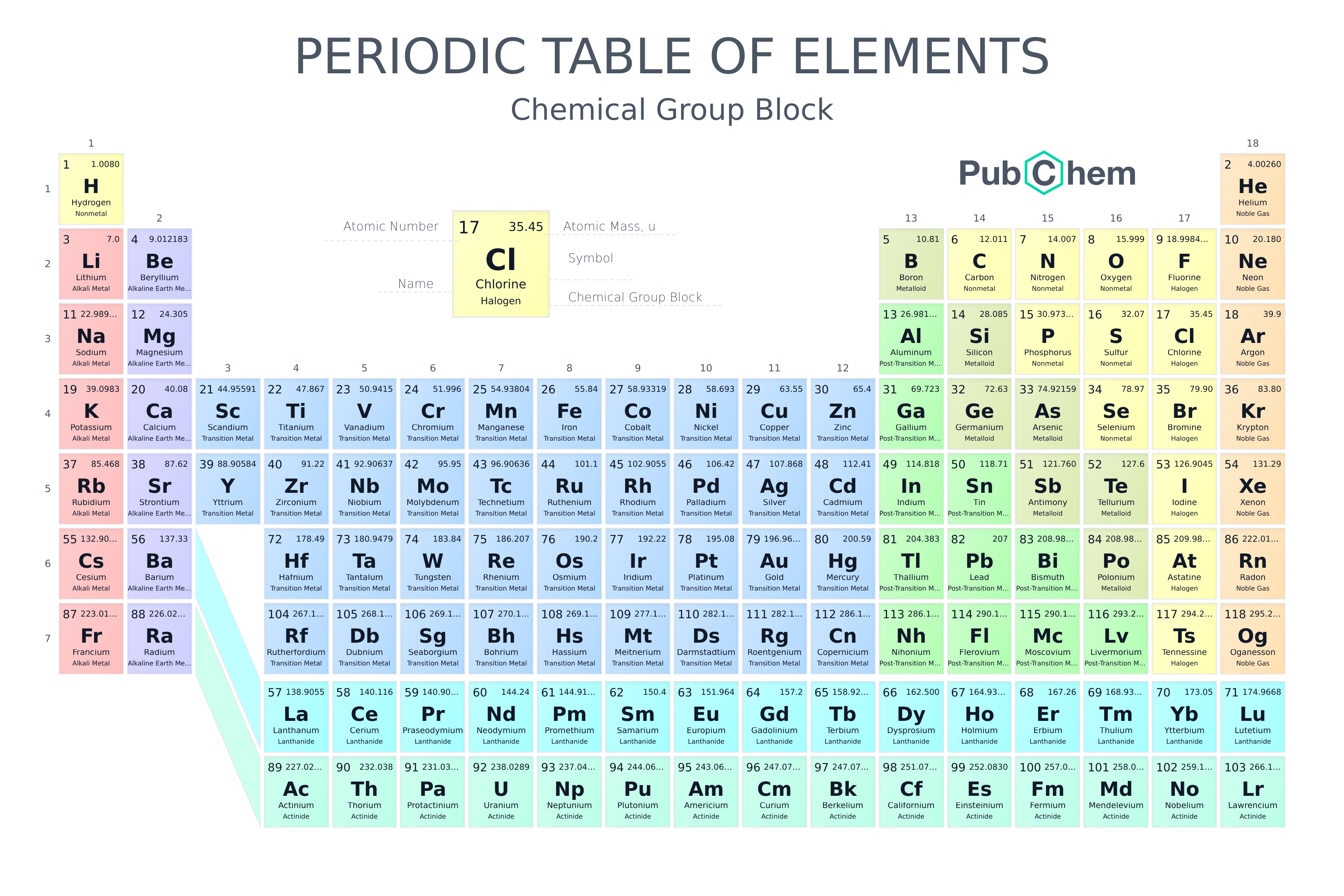
Elements are arranged in the periodic table based on various patterns. For example, elements found in the rows near the top A. have higher atomic numbers than the elements in the rows near the bottom. B. all have the same atomic
Science -CHECK MY WORK-
a.) How can you describe the location of vanadium (V, atomic number 23) on the periodic table? Choose all that apply. 1. period 5, group 4 2. period 4, group 5 3. lanthanum series 4. nonmetals 5. transition metals b.) What is true
physics
4. What is the electric potential at the surface of a gold atom’s nucleus, which is assumed to be spherical? The radius of a gold nucleus is 6.6 x 10-15 meters and the atomic number of gold is 79.
Science
1. Which statement about subatomic particles is NOT true? (1 point) Protons and neutrons have almost the same mass. Protons and electrons have opposite charges. Unlike protons and electrons, neutrons have no charge. Protons and
You can view more similar questions or ask a new question.
List Of Elements By Atomic Number
Question: The atomic mass of gold (au) is 198, and the atomic number of gold is 79. How many neutrons does a neutral atom of gold contain? Periodic Table of Elements - The periodic table is a very useful listing of all 118 elements by symbol, atomic number, and atomic mass and molecular mass. Elements with similar chemical properties are called groups. Visit BYJUS to learn more about it.